Clinical research is one of the missions of the ICM. Clinical trials make it possible to use tomorrow's drugs or therapeutic strategies today.
Objectives of clinical research
Clinical research aims to advance and improve, while respecting ethics, therapeutic management, care techniques, diagnosis or prevention through the development and implementation of innovative research projects.
Clinical trials make it possible to assess:
- a new drug (or a new therapeutic combination),
- a new way of administering them (by tablets rather than by injection for example)
- innovative treatment techniques (radiotherapy or surgery) or diagnosis (biological test for example).
Their purpose is to determine whether these new treatments are effective, well tolerated and likely to be, in the long term, offered to all the patients concerned.
Clinical trials are essential steps in making a new treatment available: it is the evaluation and validation in humans of treatments identified as promising following preclinical studies carried out in the laboratory.
Conduct of a clinical trial
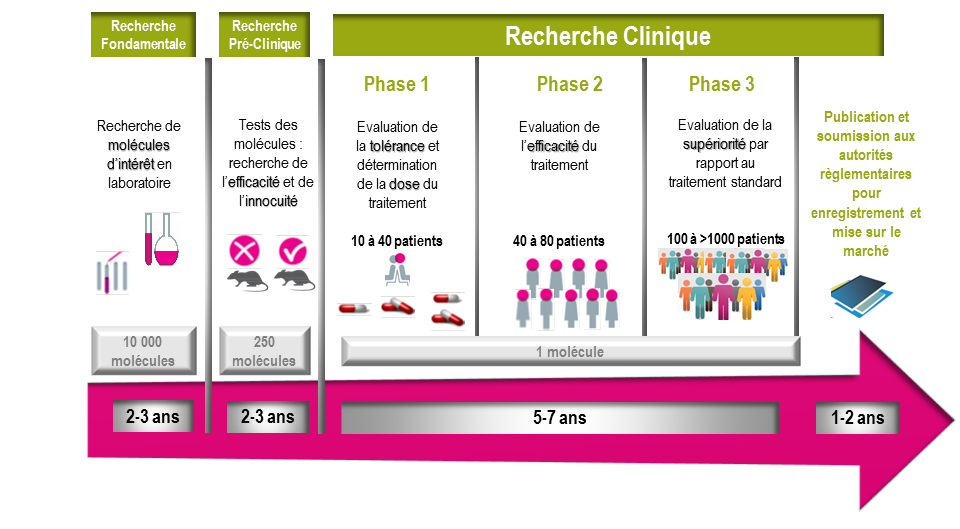 It usually takes more than 10 years for a molecule of interest to be prescribed to a patient. Clinical trials allow us to:
It usually takes more than 10 years for a molecule of interest to be prescribed to a patient. Clinical trials allow us to:
- Determine the dose and assess the tolerance of the product,
- Evaluate its effectiveness,
- Finally, to demonstrate a higher efficiency of this new treatment compared to the reference treatment.
Qu'est-ce qu'un essai clinique en oncologie ?
Regulation in clinical research
Clinical trials are subject to specific regulations and are conducted according to rigorous scientific protocols. They are regulated by laws relating to public health policy and biomedical research. The Personal Protection Committees (CPP) and the National Agency for the Safety of Medicines and Health Products (ANSM) are responsible for monitoring the relevance of the project (scientific and medical interest) and the protection of the people who will participate in it.
Participation in a clinical trial is free and voluntary (Huriet-Sérusclat Law No. 88-1138 of 12/20/1988).
After approval from the health authorities, the clinical trials selected by the ICM doctors are validated at the ICM by the internal Commission for Methodology in Clinical Research (COMERE) before being set up. Translational research projects, the creation of collections and the transfer of biological resources are submitted for the opinion of the Translational Research Committee (CORT).
Download the quality policy

Clinical research integrated into your care path at the ICM
Clinical trials are part of the daily medical activity of the ICM. They are aimed at all disciplines related to cancer therapy (surgery, radiotherapy, medical oncology, imaging, etc.) as well as care provided during or after oncological treatments (psycho-oncology, physical activity, physiotherapy, hypnosis … called supportive care). They are offered by doctors to their patients as soon as possible. The care path is then coordinated by a Clinical Research Associate (ARC), while remaining integrated into the overall care of the ICM.
Participation in a clinical trial
You may be offered participation in clinical studies during your care at the ICM. It often provides access to therapeutic innovations. If you decide to no longer participate in a trial, you can leave it of your own accord and receive another treatment adapted to your disease. This decision will not call into question the commitment of the medical team to treat you.

Agreeing to participate in a clinical trial is like challenging yourself. I feel surrounded, there is always someone to answer my questions. It's reassuring.
Clinical research explained in video
Organization of clinical research at the ICM
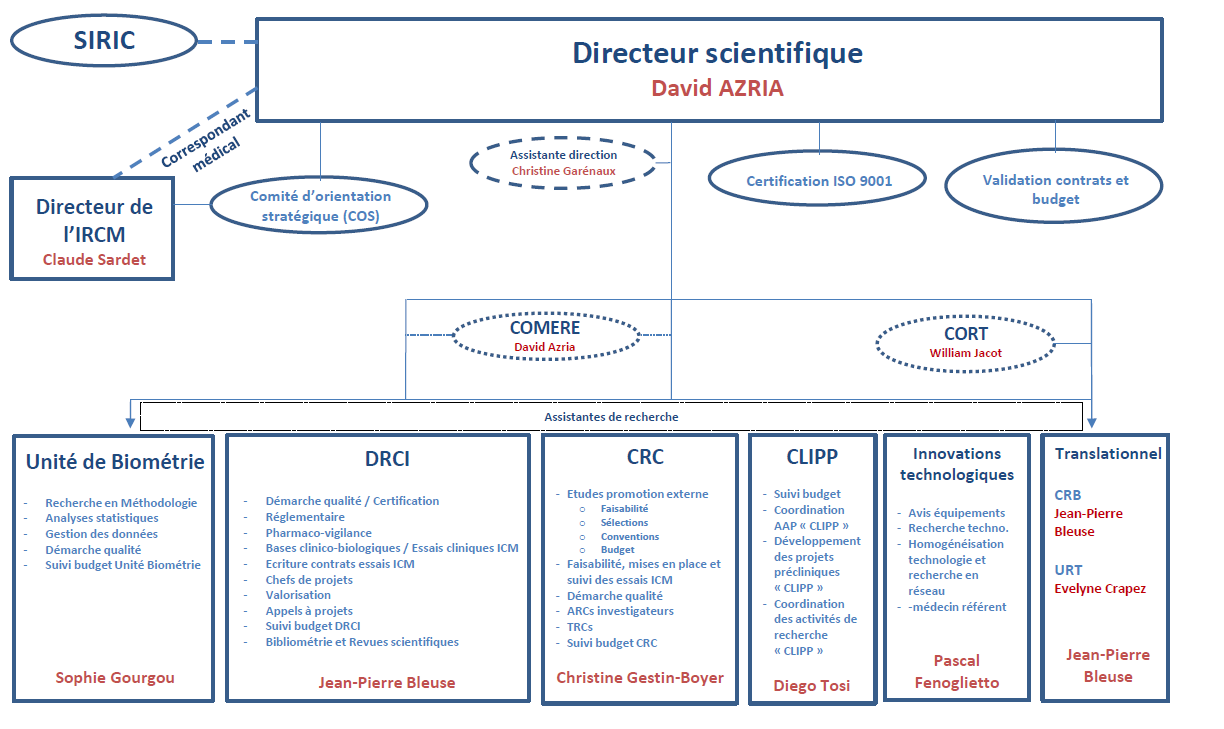
The activity of promoting clinical research at the ICM is carried out by the DRCI (Department of Clinical Research and Innovation).
The investigative activity of clinical research is carried out by the CRC (Clinical Research Center).
As a CLCC, the Institute combines basic, translational and clinical research to develop integrated, patient-centered cancer research.
Thus, the clinical research units work in close collaboration with the biometrics, CLIPP, CRB, URT and technological innovation teams and are grouped together within a single department: the Scientific Department.
Within the framework of Montpellier Cancer, the ICM is one of 8 centers in France labeled Site de Recherche Integrated en Cancerologie (SIRIC) by the National Cancer Institute (INCa).

"De la Recherche au Patient"
L'ICM vous propose de découvrir la réalité des équipes de recherche clinique au travers de photographies montrant leur quotidien et leur contribution à l'innovation thérapeutique en cancérologie au service de nos patients.
Ce reportage a été réalisé par Sophie Pattingre, chercheuse à l'IRCM et passionnée de photographie.
us propose de découvrir la réalité des équipes de recherche clinique au travers de cette exposition de photographies.

ISO 9001 certification
The ICM Clinical and Biometric Research units have been officially ISO 9001 certified by AFNOR Certification since August 2017.
ISO 9001 certification is an international standard that defines requirements for the implementation of a Quality Management System (QMS). This is granted for a period of 3 years. Internationally recognized, the ISO 9001 standard guarantees a high level of quality and safety in project management for everyone, patients, doctors and partners.
ICM certified
The ICM is, today, the only establishment in the Occitanie Mediterranean region to obtain this certification which mobilized more than 90 people for more than a year.
A quality policy has been drawn up in line with the strategic orientations set out in each of the clinical research and biometrics sectors, along 4 axes. It testifies to the desire of the Management and the heads of the Units to implement a continuous improvement process, with the following challenges:
- Meet the expectations of customers and interested parties
- Continuously improve the organization of units
- To allow the General Management to have visibility on the approach of the units.


